Recombinant Human G/T mismatch-specific thymine DNA glycosylase (TDG)
In Stock-
中文名称:人TDG重组蛋白
-
货号:CSB-EP623816HU
-
规格:¥1836
-
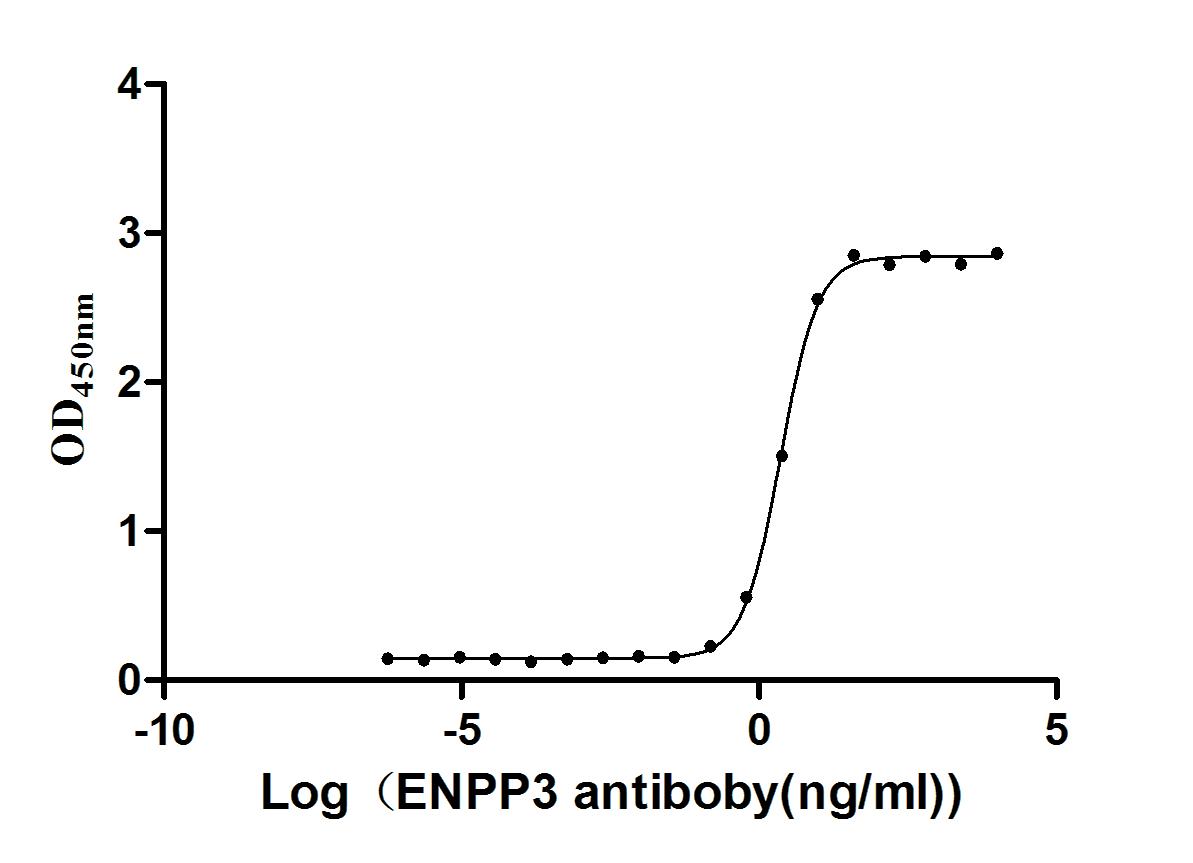
图片:
-
其他:
产品详情
-
纯度:Greater than 90% as determined by SDS-PAGE.
-
基因名:
-
Uniprot No.:
-
种属:Homo sapiens (Human)
-
蛋白长度:Full Length
-
来源:E.coli
-
分子量:52.1 kDa
-
表达区域:1-410aa
-
氨基酸序列MEAENAGSYSLQQAQAFYTFPFQQLMAEAPNMAVVNEQQMPEEVPAPAPAQEPVQEAPKGRKRKPRTTEPKQPVEPKKPVESKKSGKSAKSKEKQEKITDTFKVKRKVDRFNGVSEAELLTKTLPDILTFNLDIVIIGINPGLMAAYKGHHYPGPGNHFWKCLFMSGLSEVQLNHMDDHTLPGKYGIGFTNMVERTTPGSKDLSSKEFREGGRILVQKLQKYQPRIAVFNGKCIYEIFSKEVFGVKVKNLEFGLQPHKIPDTETLCYVMPSSSARCAQFPRAQDKVHYYIKLKDLRDQLKGIERNMDVQEVQYTFDLQLAQEDAKKMAVKEEKYDPGYEAAYGGAYGENPCSSEPCGFSSNGLIESVELRGESAFSGIPNGQWMTQSFTDQIPSFSNHCGTQEQEEESHA
Note: The complete sequence including tag sequence, target protein sequence and linker sequence could be provided upon request. -
蛋白标签:N-terminal 10xHis-tagged
-
产品提供形式:Liquid or Lyophilized powder
Note: We will preferentially ship the format that we have in stock, however, if you have any special requirement for the format, please remark your requirement when placing the order, we will prepare according to your demand. -
缓冲液:If the delivery form is liquid, the default storage buffer is Tris/PBS-based buffer, 5%-50% glycerol. If the delivery form is lyophilized powder, the buffer before lyophilization is Tris/PBS-based buffer, 6% Trehalose.
-
复溶:We recommend that this vial be briefly centrifuged prior to opening to bring the contents to the bottom. Please reconstitute protein in deionized sterile water to a concentration of 0.1-1.0 mg/mL.We recommend to add 5-50% of glycerol (final concentration) and aliquot for long-term storage at -20℃/-80℃. Our default final concentration of glycerol is 50%. Customers could use it as reference.
-
储存条件:Store at -20°C/-80°C upon receipt, aliquoting is necessary for mutiple use. Avoid repeated freeze-thaw cycles.
-
保质期:The shelf life is related to many factors, storage state, buffer ingredients, storage temperature and the stability of the protein itself.
Generally, the shelf life of liquid form is 6 months at -20°C/-80°C. The shelf life of lyophilized form is 12 months at -20°C/-80°C. -
货期:3-7 business days
-
注意事项:Repeated freezing and thawing is not recommended. Store working aliquots at 4°C for up to one week.
-
Datasheet & COA:Please contact us to get it.
相关产品
靶点详情
-
功能:DNA glycosylase that plays a key role in active DNA demethylation: specifically recognizes and binds 5-formylcytosine (5fC) and 5-carboxylcytosine (5caC) in the context of CpG sites and mediates their excision through base-excision repair (BER) to install an unmethylated cytosine. Cannot remove 5-hydroxymethylcytosine (5hmC). According to an alternative model, involved in DNA demethylation by mediating DNA glycolase activity toward 5-hydroxymethyluracil (5hmU) produced by deamination of 5hmC. Also involved in DNA repair by acting as a thymine-DN...显示更多
-
基因功能参考文献:
- TET2- and TDG-mediated changes are required for the acquisition of distinct histone modifications in divergent terminal differentiation of myeloid cells. PMID: 28973458
- Results indicate how Thymine DNA glycosylase (TDG) employs an adaptable active site to excise a broad variety of nucleobases from DNA. PMID: 27805810
- Results indicate the presence of base excision repair -dependent and base excision repair-independent functions of TDG, which are involved in regulation of cellular DNA damage responses and gene expression patterns. PMID: 28318075
- findings indicate that sumoylation and SUMO binding are not essential for TDG-mediated excision and repair of 5-carboxylcytosine bases. PMID: 26917720
- Data show that Ras protein regulates inhibitor of growth protein 4 (ING4)-thymine-DNA glycosylase (TDG)-Fas protein axis to promote apoptosis resistance in pancreatic cancer. PMID: 26544625
- A long TA repeat in the promoter region of IL28B was associated with spontaneous HCV clearance. PMID: 25735432
- NEIL1 and NEIL2 cooperate with TDG during base excision: TDG occupies the abasic site and is displaced by NEILs, which further process the baseless sugar, thereby stimulating TDG-substrate turnover. PMID: 26751644
- Computational modeling study investigated the glycosidic bond cleavage reaction in human thymine DNA glycosylase PMID: 26320595
- levels of TET3 and TDG mRNAs were independent prognostic factors for early breast cancer patients who received anthracycline chemotherapy PMID: 26207381
- TDG releases the excised base from its tight product complex with abasic DNA, contrary to previous reports. Moreover, DNA-free TDG exhibits no significant binding to free nucleobases (uracil, thymine, 5-hydroxymethyluracil) PMID: 26358812
- these results suggest that individuals harboring the G199S in Thymine DNA glycosylase variant may have increased risk for developing cancer. PMID: 25375110
- Data indicate that both thymine-DNA glycosylase (hTDG) and a second glycosylase, hOGG1, recognized structurally different 8-oxoguanine lesions. PMID: 25712093
- TDG, as a new coactivator, promotes beta-catenin/TCFs transactivation and functionally cooperates with CBP in canonical Wnt signaling. PMID: 24748645
- CRL4(Cdt2)-dependent degradation of TDG occurs in S phase because of the requirement for TDG to interact with chromatin-loaded PCNA, and this degradation is important for preventing toxicity from excess TDG. PMID: 24962565
- Results show TARID binds to the TCF21 promoter and recruits GADD45A and TDG to direct base excision repair for demethylation. PMID: 25087872
- Whereas sumoylation substantially weakens TDG binding to DNA, TDG approximately SUMO-1 still binds relatively tightly to AP-DNA (Kd approximately 50 nM). PMID: 24753249
- these findings provide insights into the in vivo dynamics of TDG SUMOylation and further clarify the TDG-RNF4 interaction. PMID: 24727457
- Thymine DNA glycosylase is a positive regulator of Wnt signaling in colorectal cancer PMID: 24532795
- provide evidence for the existence of a functional ternary complex containing TDG, CBP and activated RARalpha PMID: 24394593
- SIRT1 affects DNA repair through binding to thymine DNA glycosylase (TDG), stimulating TDG glycosylase activity, maintaining TDG in a hypoacetylated state, and regulating TDG expression PMID: 23952905
- Results imply that 5-carboxylcytosine (5caC) can adopt alternative conformations (either N157-interacting or N230-interacting) in the thymine DNA glycosylase active site to interact with either of the two asparagine side chain for 5caC excision. PMID: 23680598
- TDG 3'untranslated region (UTR) contains two miR-29 binding sites; the miR-29 mimic decreases TDG mRNA by 40%, while miR-29 inhibitor increases TDG mRNA by 43.7% in human vascular smooth muscle cell cultures. PMID: 23820384
- Human thymine-DNA glycosylase is able to excise 8oxoA in 8oxoA*T pairs. PMID: 23209024
- A structural study of catalysis by the thymine DNA glycosylase catalytic domain. PMID: 22962365
- genetic variants in TDG, important not only in base excision repair but also in regulating the epigenome and gene expression, which may contribute to the non-melanoma skin cancer associated increase in overall cancer risk. PMID: 22581838
- We solved a crystal structure of TDG (catalytic domain) bound to a substrate analog and characterized active-site residues by mutagenesis, kinetics, and molecular dynamics simulations. PMID: 22573813
- 5-carboxylcytosine is specifically recognized in the active site of thymine DNA glycosylase PMID: 22327402
- Thymine DNA glycosylase can rapidly excise 5-formylcytosine and 5-carboxylcytosine: potential implications for active demethylation of CpG sites. PMID: 21862836
- DNMT3L exerts a major effect on the transcriptional regulation of a specific target gene, such as thymine DNA glycosylase PMID: 20428781
- Studies lead to the characterization of a small structural domain in the TDG N-terminal region preceding the catalytic core and coinciding with the region of functional regulation of TDG's activities. PMID: 18512959
- the TDG-NCoA-3 interaction is important for broad range activation of steroid hormone nuclear receptors PMID: 19652917
- role in removing thymine produced by deamination of 5-methylcytosine and not removal of ethenocytosine PMID: 12493755
- Xeroderma pigmentosum group C protein interacts physically and functionally with this enzyme PMID: 12505994
- thymine-DNA glycosylase potentiates transcription of estrogen-regulated genes through direct interaction with estrogen receptor alpha PMID: 12874288
- Polymorphisms in thymine DNA Glycosylase is associated with lung Neoplasms PMID: 15225156
- unique range of each TDG activity corresponding to the three fractions indicates that human cells possibly express three distinct TDGs PMID: 15668625
- Upon DNA interaction, TDG undergoes a dramatic conformational change, which involves its flexible N-terminal domain and accounts for its nonspecific DNA binding ability during base excision repair. PMID: 15823533
- structure of the central region of human TDG conjugated to SUMO-1 at 2.1 A resolution PMID: 15959518
- Results describe the crystal structure of the central region of thymine-DNA glycosylase conjugated to SUMO-3. PMID: 16626738
- A novel missense variant A196G was found in familial colorectal cancer DNA suggesting a limited role for this gene in the devlopment of CRC. PMID: 17029639
- TDG sumoylation promotes intramolecular interactions with amino- and carboxy-terminal SUMO-1 binding motifs that dramatically alter the biochemical properties and subcellular localization of TDG PMID: 17060459
- The ability of human thymine-DNA glycosylase (TDG) to excise 8-(hydroxymethyl)-3,N(4)-ethenocytosine (8-hm-varepsilonC) and 3,N(4)-ethanocytosine (EC) was investigated and compared with varepsilonC, a known substrate for TDG. PMID: 17270163
- analysis of 5-halogenated uracils in human thymine DNA glycosylase PMID: 17602166
- Thymine DNA glycosylase activity is significantly stimulated by hHus1, hRad1, hRad9 separately, and by the 9-1-1 complex. PMID: 17855402
- Expression of exogenous enzyme can functionally compensate for lower repair activities of damaged DNA in a myeloma cell line. PMID: 17965616
- A crystal structure of hTDG (catalytic domain, hTDG(cat)) in complex with abasic DNA, at 2.8 A resolution, is reported. PMID: 18587051
- Apurinic/apyrimidinic endonuclease 1 actively stimulates thymine DNA glycosylase by disrupting the product complex PMID: 18805789
- These observations suggest that TDG modulates the biological function of p53 and other members of the p53 family as a transcriptional coactivator. PMID: 18951877
- There was a 1.198-fold increased micronucleus frequency for individuals carrying TDG 199Gly/Ser + Ser/Ser genotypes compared with those carrying Gly/Gly genotype (P < 0.05) for exposure to vinyl chloride. PMID: 19369898
- excision of DNA-incorporated 5-FU by TDG generates persistent DNA strand breaks, delays S-phase progression, and activates DNA damage signaling PMID: 19402749
收起更多
-
亚细胞定位:Nucleus.
-
蛋白家族:Uracil-DNA glycosylase (UDG) superfamily, TDG/mug family
-
数据库链接:




-AC1.jpg)











